#ISO 13485 INTERNAL AUDIT CHECKLIST ISO#
A medical device manufacturer that has prepared for ISO 13485 certification by building its quality management system will want to comprehensively assess for gaps before proceeding with a second-party audit that could lead to certification. This is considered a high-stakes audit because the auditor serves as a registrar (or accredited certification body) that determines ISO certification status. It is recommended that buyers should regularly perform monitoring audits on their critical suppliers.Ī second-party organization audits for compliance to ISO standards and awards the certification (or “registration” in ISO’s terminology). This requires the organization and its employees/operators to follow procedures and continuously improve their own system. It is merely a framework that guides the organization in deploying best practices. The ISO 13485 QMS is not a one-time event.

Many factories initially seem to have a high quality capability but over time their performance declines.
Medical device manufacturers increasingly rely on suppliers for specialized materials, software, packaging, and labeling. Onboarding and ensuring a potential supplier’s compliance with the standard.All this amounts to a dynamic picture that requires continual vigilance.Ī medical device manufacturer may need an ISO 13485 Internal Audit Assessment when: The ISO 13485 standard is updated periodically. Medical device organizations grow and evolve quickly. When and why is an ISO 13485 Internal Audit Assessment needed? This can include a medical device maker, supplier, external testing organization, and others. It is important to note that the Internal Audit Assessment can be applied to any organization in the medical device supply chain that needs to demonstrate the suitability of its quality management system for customer and regulatory requirements. The checklist may be used as a tool for the audit, and it may be presented, along with an audit summary, as a product of the analysis. The auditor goes through each requirement of the standard and compares it to the current state in the medical device organization.
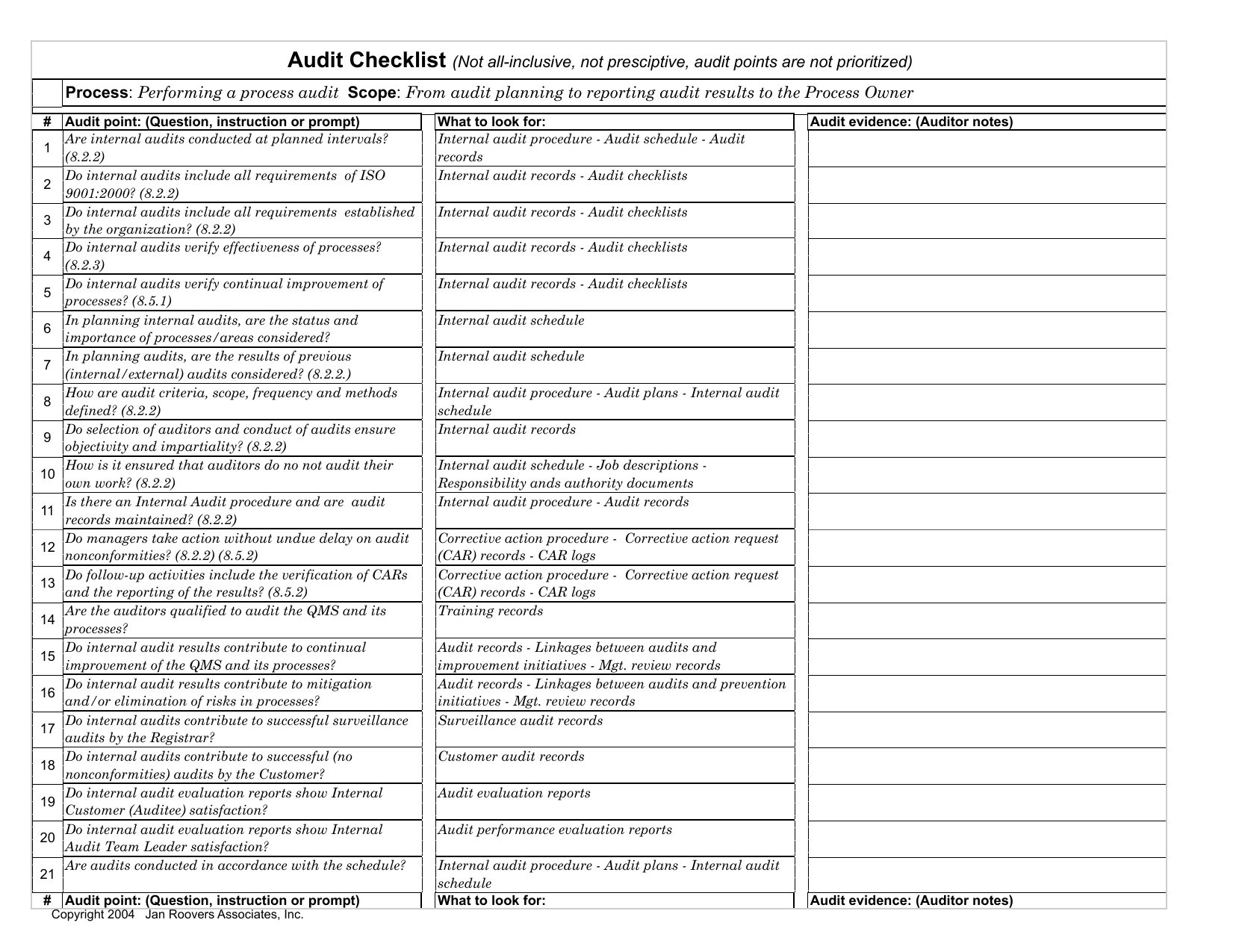
This is the current revision of the medical device quality management system standard for medical device firms published by the International Organization for Standardization.Įssentially, this analysis can be condensed into a checklist. The analysis will show where the organization is and is not meeting the standard.įor a medical device manufacturer, an ISO 13485 Internal Audit Assessment will systematically compare the current quality management system to the requirements in ISO 13485:2016. Agenda and checklist for a third-party ISO 13485 audit Introduction – ISO 13485 Internal Audit Assessment What is an Internal Audit Assessment?Īn Internal Audit Assessment is a formal, comprehensive comparison of the current state of an organization’s processes and procedures against a standard or regulation.


 0 kommentar(er)
0 kommentar(er)
